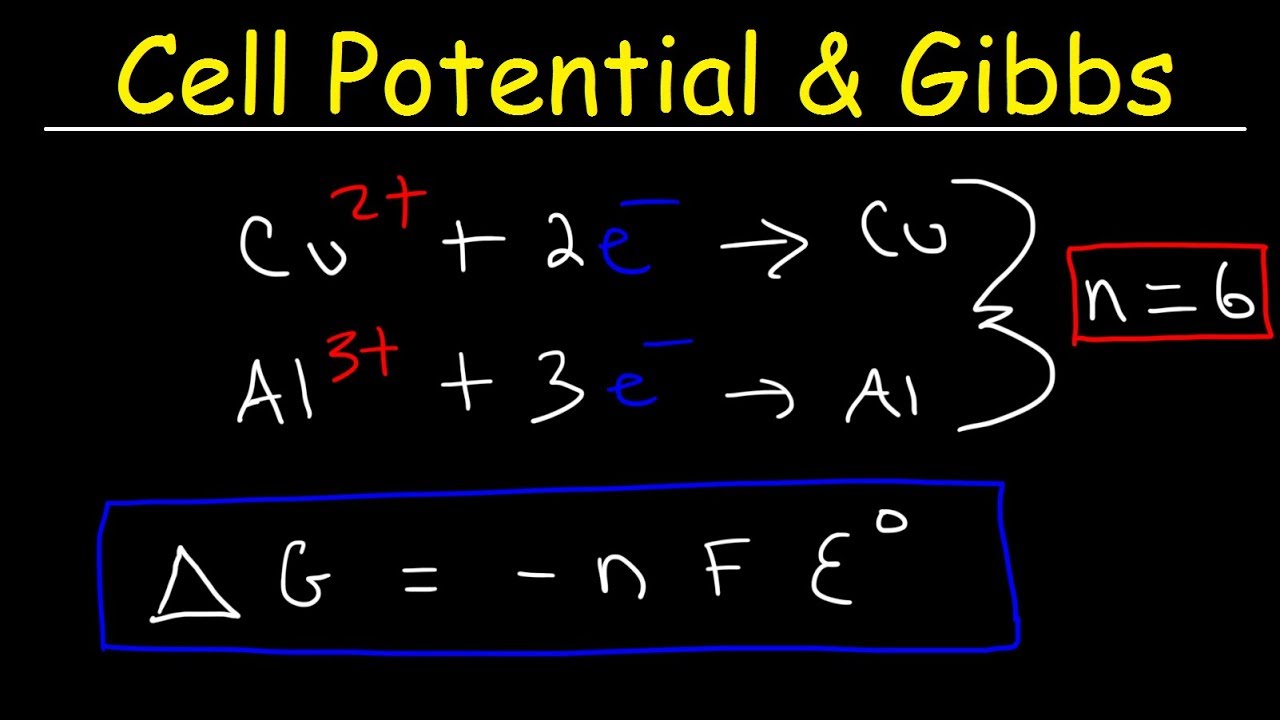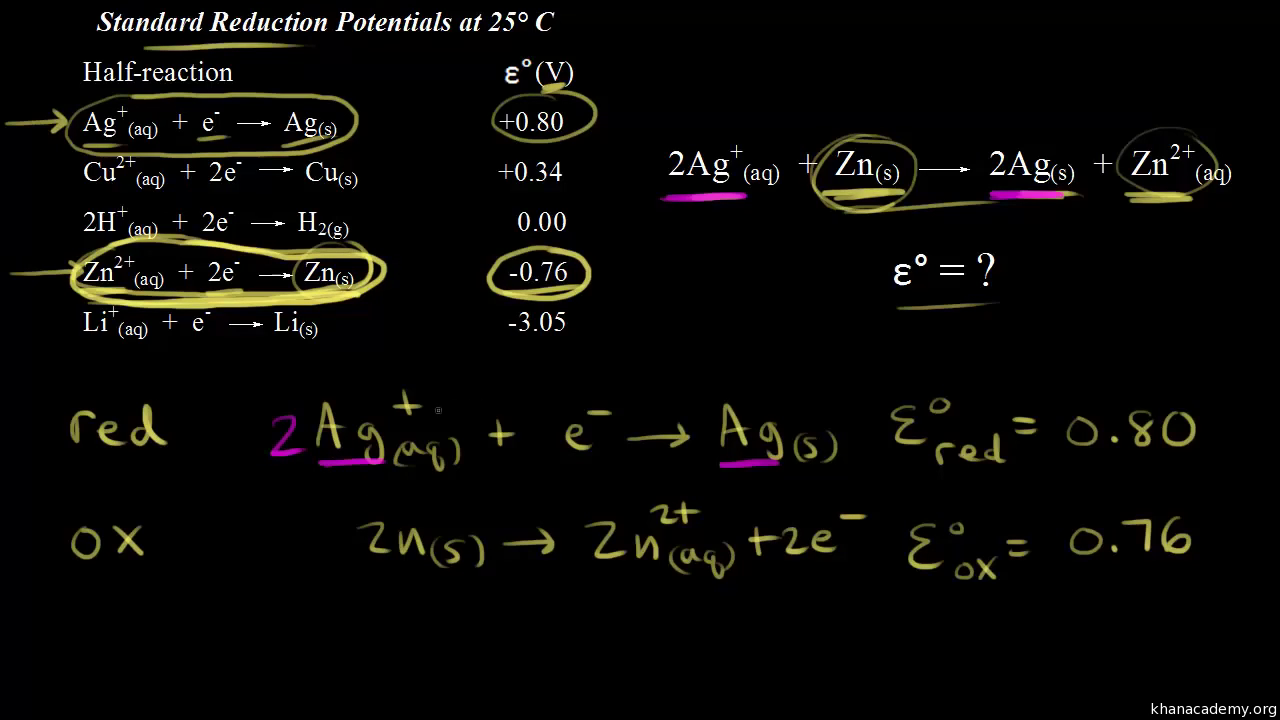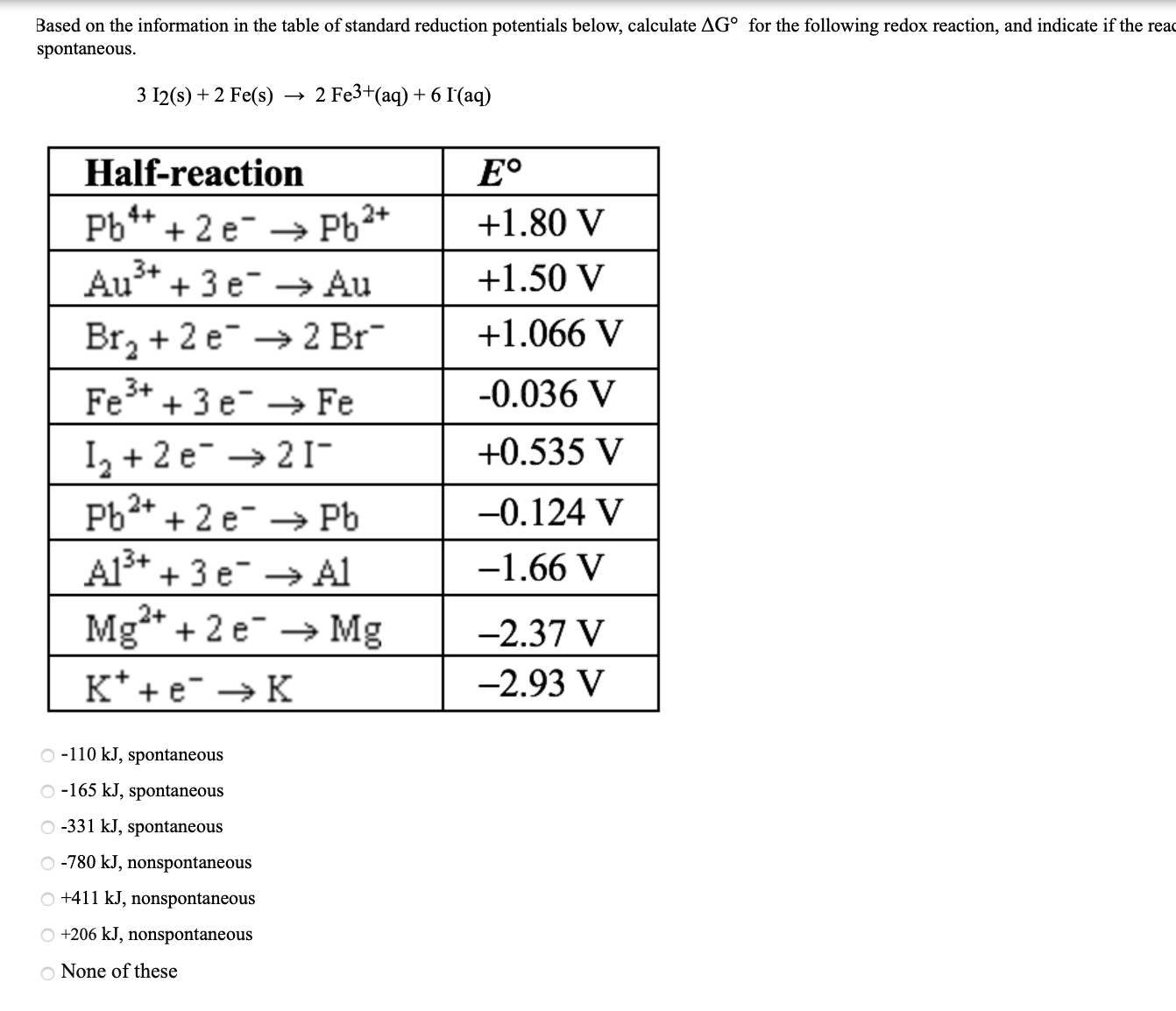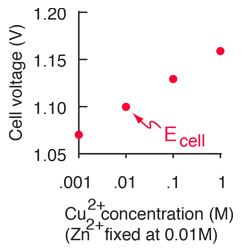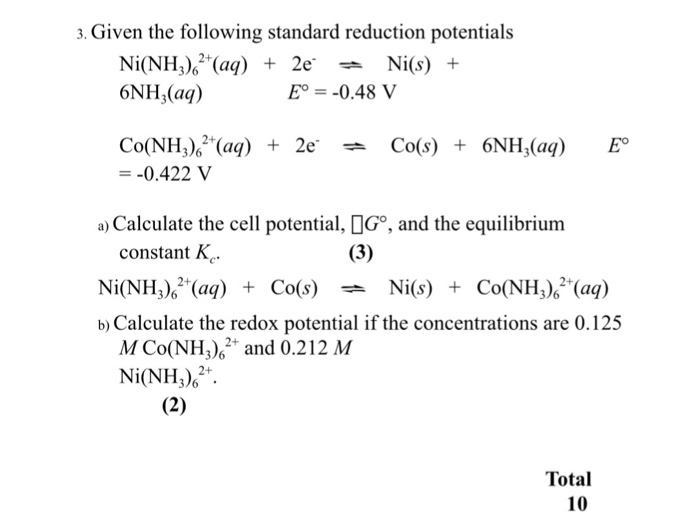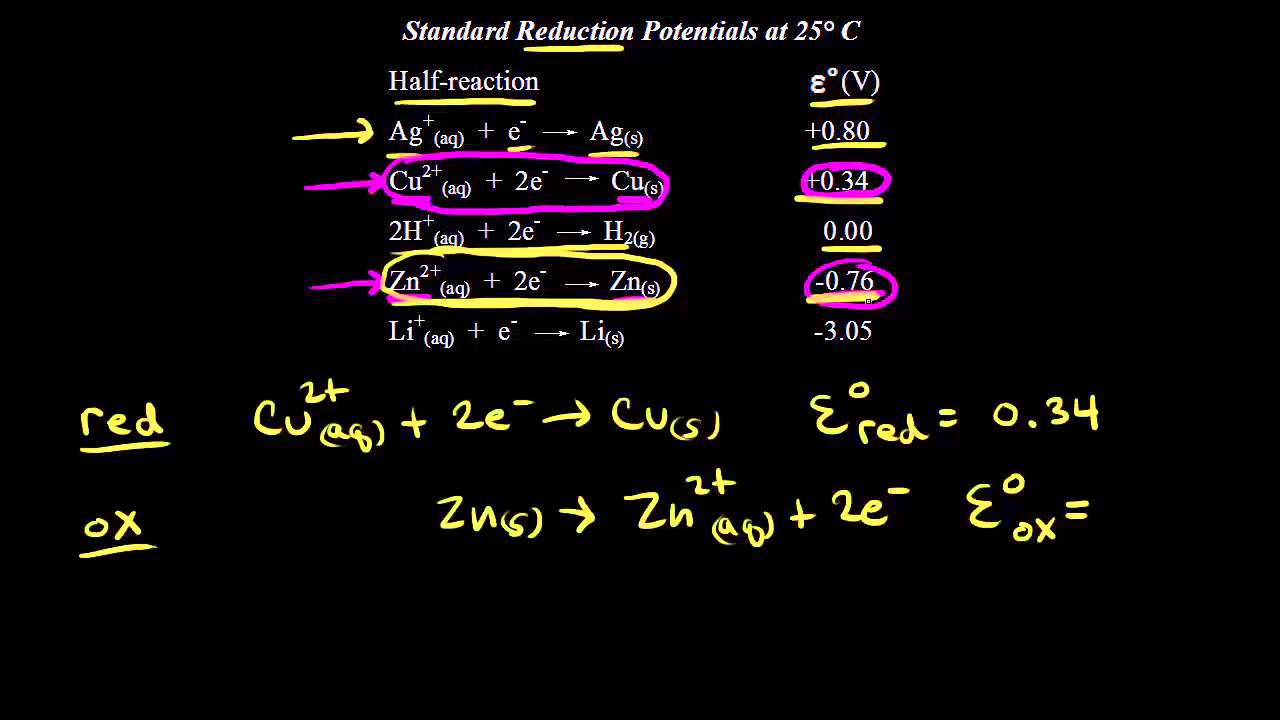
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube

equilibrium - Calculate the cathode electrode potential in this redox reaction - Chemistry Stack Exchange
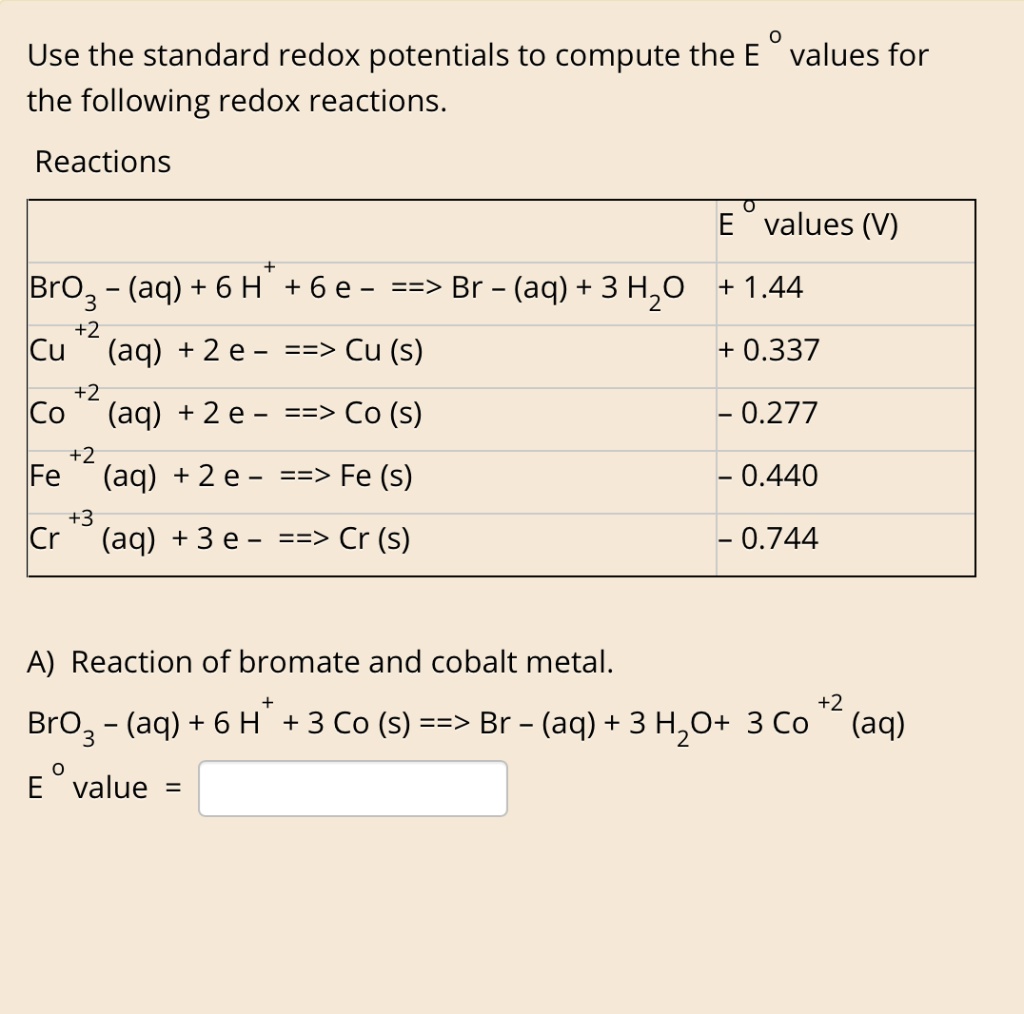
SOLVED: Use the standard redox potentials to compute the E values for the following redox reactions. Reactions values BrO3 (aq) + 6 H +6 e - ==> Br - (aq) + 3
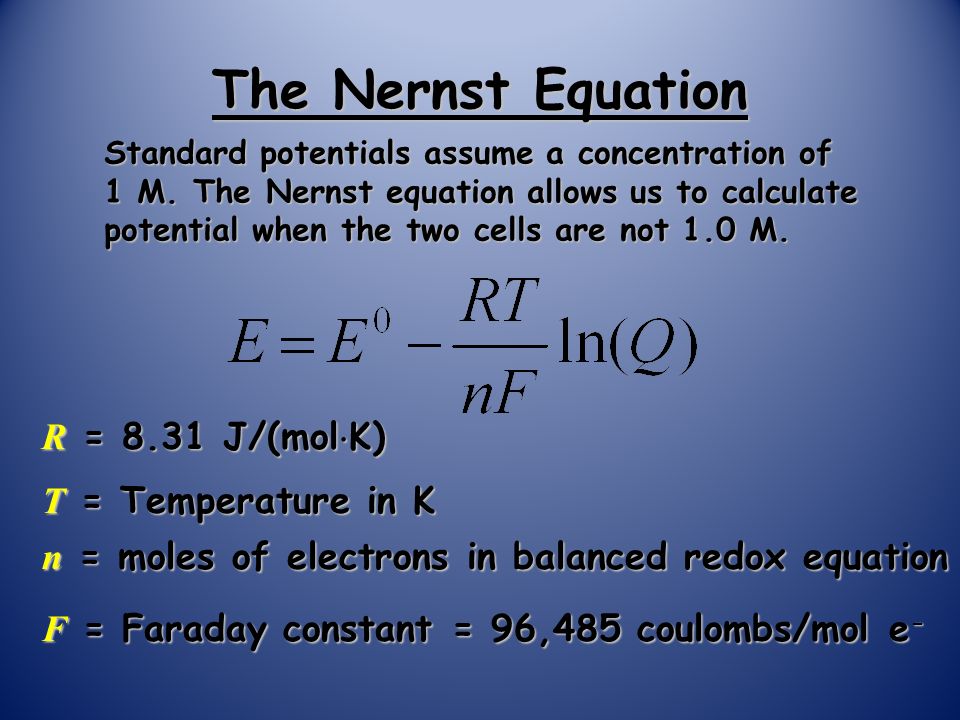
The Nernst Equation Standard potentials assume a concentration of 1 M. The Nernst equation allows us to calculate potential when the two cells are not. - ppt download

Standard reduction potential of aluminum, calcium, iron, indium and... | Download Scientific Diagram

Given standard electrode potentials: Fe^3 + + 3e^-→ Fe;E^0 = - 0.036V Fe^2 + + 2e^-→ Fe;E^0 = - 0.440V The standard electrode potential E^o for Fe^3 + + e^ - → Fe^2 + is:

Species-Specific Standard Redox Potential of Thiol-Disulfide Systems: A Key Parameter to Develop Agents against Oxidative Stress | Scientific Reports

Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide | Inorganic Chemistry